The Role of Liquid Sensors in Environmental Monitoring

Liquid sensors play an important role in environmental monitoring so as to safeguard our planet’s resources. From detecting pollution to maintaining water quality and promoting conservation efforts, these sensors have become indispensable tools. This article reviews what environmental monitoring is, and the role of liquid sensors in populations with limited access to clean water. What […]
Chlorine Disinfection of Water: Free Chlorine and Total Chlorine Sensors

Free and total chlorine measurements are important when determining the effectiveness of water treatment efforts. This is because they provide insight into the interaction between chlorine and contaminants, as well as help in delivering the right chlorine dosage. In this article, we review what free chlorine and total chlorine are, their sensors and their importance […]
The Dangers of Lead in Drinking Water

In this article, we review possible channels of lead contamination in drinking water, dangers of ingestion, safe levels, and detection.
Cooling Tower Water Treatment

In this article, we discuss the importance of assessing cooling tower water quality, methods of testing, and common treatment processes.
What is the pH of Drinking Water?

This article reviews the importance of pH in drinking water, optimal pH range for drinking water, how to measure it, and its health effects.
PTSA – Uses and Measurements
This article will explore PTSA uses and measurements, its uses in water treatment facilities and other applications.
New Technologies for Wastewater Treatment

Wastewater treatment plays a crucial role in preserving our environment and ensuring the availability of clean water for several purposes. As the global population increases and industries expand, the demand for effective wastewater treatment solutions is on the rise. Fortunately, emerging technologies are paving the way for innovative and sustainable approaches to address this challenge. […]
AlpHa Measurement Solutions Launches In-line PTSA Sensor Portfolio

AlpHa AlpHa Measurement Solutions, a leader in liquid-sensing technology announces the launch of its new in-line PTSA Fluorometer Portfolio.
Water Conductivity Range

Water conductivity range is a crucial parameter when assessing the quality and purity of water in various applications. Moreover, it is a measure of the ability of water to conduct electricity, which is proportional to the quantity of dissolved ions. In this article, we delve into the significance of water conductivity range, explore it for […]
Saturated Calomel Electrode
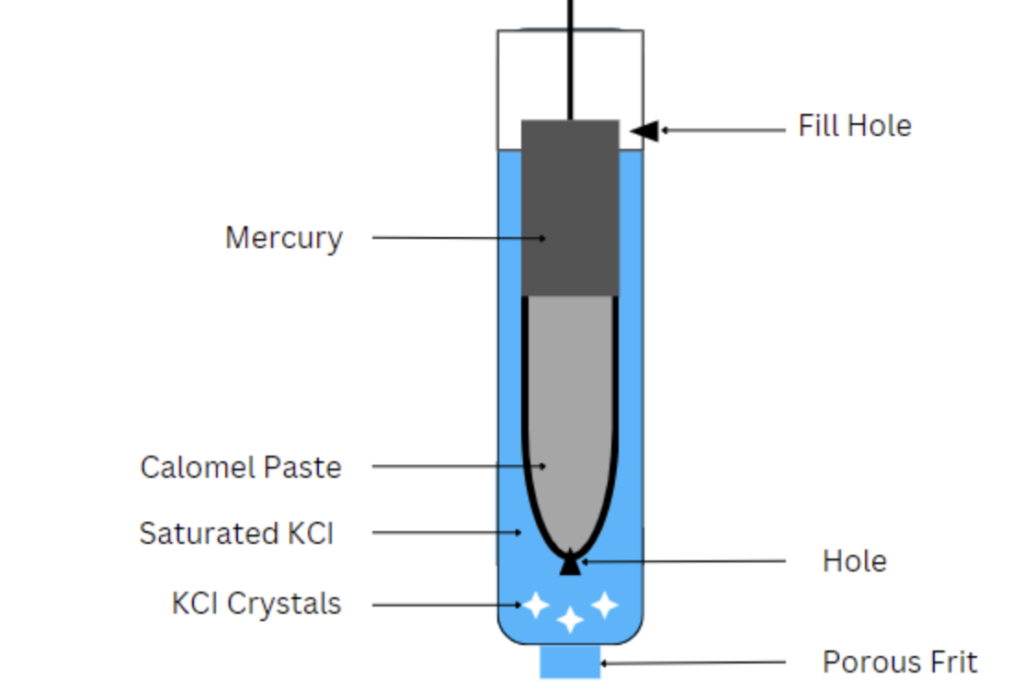
Electrodes play a pivotal role in electrochemical measurements, and one of the stalwarts in the field is the saturated calomel electrode. It is a reference electrode that functions via a reaction between elemental mercury and mercurous chloride. In this article, we review a typical diagram of a saturated calomel electrode, then delve into its theory […]

