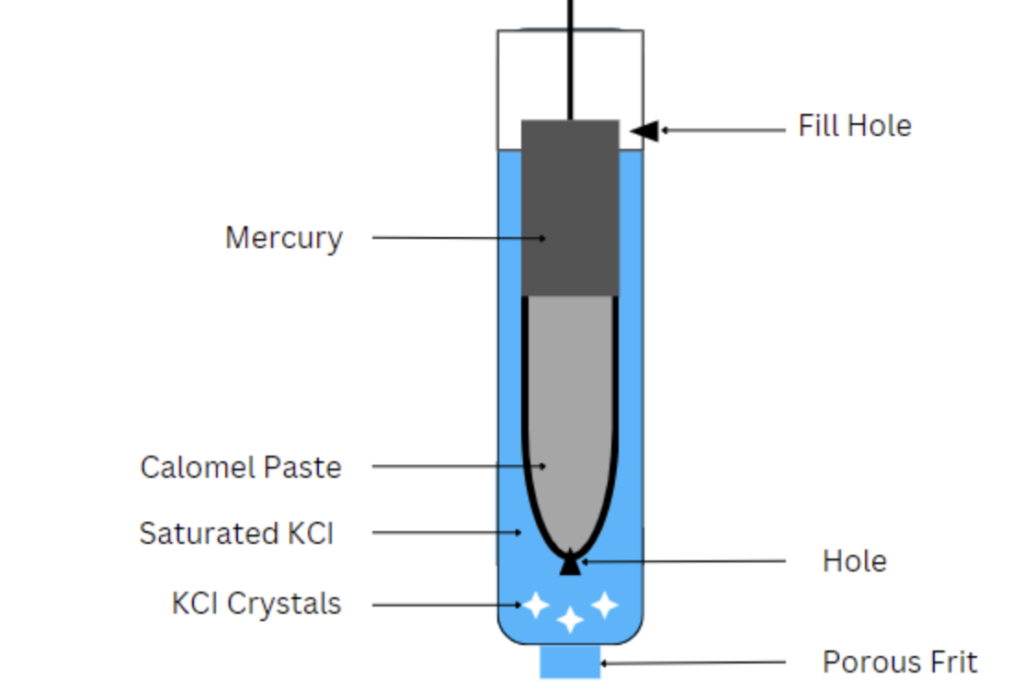
Electrodes play a pivotal role in electrochemical measurements, and one of the stalwarts in the field is the saturated calomel electrode. It is a reference electrode that functions via a reaction between elemental mercury and mercurous chloride.
In this article, we review a typical diagram of a saturated calomel electrode, then delve into its theory of operation, and compare it vs silver/silver chloride electrode.
Saturated Calomel Electrode Diagram
A saturated calomel electrode (SCE) consists of a glass vessel having a bent side tube. At the bottom of the tube is pure mercury, which is covered with calomel paste – mercury-mercurous chloride (Hg+Hg2Cl2). Mercury provides a stable and conductive medium for the electrode to operate. This choice of mercury is crucial as it exhibits low reactivity and remains liquid across a wide temperature range.
Calomel paste serves as a salt bridge that allows ion exchange while maintaining a constant chloride ion concentration. It also contributes to the SCE’s stable potential.
The remaining portion of the cell contains saturated potassium chloride (KCl). Saturated KCl solution ensures that the chloride ion concentration in the electrode remains constant and prevents deviations in potential over time.
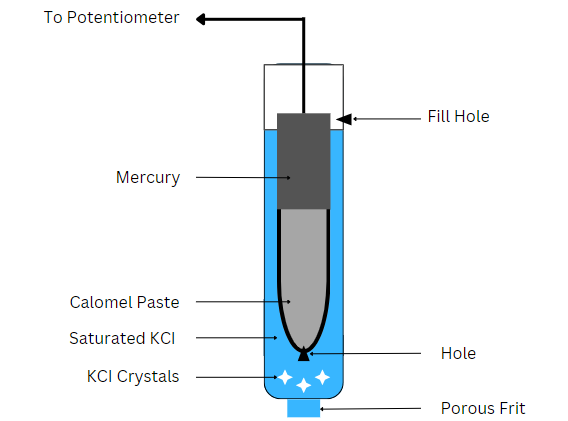
Generally, a platinum wire immersed in the mercury layer allows contact with an external circuit such as a potentiometer. The side tube induces electrical contact with a salt bridge. Contact to the measurement cell occurs via a porous glass frit or fiber. This allows for ion movement, but not the bulk solution.
The SCE has a stable reference potential, with an E0 value of 0.242 V. Practical application of the standard hydrogen electrode (SHE) is limited by the difficulty in preparing and maintaining the electrode, especially because of the requirement for hydrogen gas in the half-cell. Thus, it makes the saturated calomel electrode a preferable choice in various electrochemical applications.
Theory of Operation
A saturated calomel electrode (SCE) often serves as a secondary reference electrode. Depending on the nature of the other electrode in the galvanic cell, the SCE acts either as an anode or cathode. When functioning as an anode, the reaction that occurs is an oxidation reaction, where mercury first oxidizes to mercuric ions.
![]()
Then, the chloride ions supplied by the KCl solution combine with the mercuric ions to form insoluble mercurous chloride.
![]()
On the other hand, if the SCE serves as a cathode in the galvanic cell, a reduction reaction occurs.
![]()
Advantages
The use of SCE comes alongside several advantages including:
- Easy set-up and reproduction.
- Convenient transportation.
- Stable potential that does not change significantly over time nor with slight temperature variations.
- It utilizes less auxiliary assets. The SCE comes with a side tube containing a KCl solution, so there is no requirement for a separate salt bridge. Also, the KCl solution is refillable and as such is less prone to reference junction blockage.
Applications of Saturated Calomel Electrode
Due to the stability of the potential of saturated calomel electrode, its applications include:
- Cyclic Voltammetry: In this application, SCE serves in conjunction with a working electrode to study the electrochemical properties of an analyte in solution, or of a molecule that is absorbed onto the electrode.
- Electrochemistry: The SCE serves in establishing a relationship between electrical potential difference and identifiable chemical changes.
- Measurement of pH: SCE can serve in measuring pH of solutions.
Saturated Calomel vs Silver/Silver Chloride Electrode
Both saturated calomel and silver/silver chloride serve as reference electrodes and are immersed in a solution saturated with potassium chloride. Also, the activity of metal ions in both electrodes is fixed by the solubility of the metal salt. Although they serve in similar applications, there some differences exist as the following table highlights.
| Parameter | Saturated Calomel | Silver/Silver Chloride |
| Composition | Comprises mercury, calomel paste, and saturated potassium chloride solution. | Consists of a silver wire coated with silver chloride. The electrolyte could be potassium chloride or sodium chloride. |
| Reference Potential | Maintains a reference potential of about 0.242 V vs the standard hydrogen electrode. | Typically has a reference potential of about 0.197 V vs standard hydrogen electrode. |
| Environment | The presence of mercury poses a health hazard. Also, there could be contamination issues. | It is environmentally friendly as it does not contain mercury. |
| Stability | Offers a stable and reproducible reference potential. | Prone to chloride ion depletion which affects long term stability. |
| Temperature | Could be affected by higher temperatures. | More stable than the saturated calomel electrode at higher temperatures. |
| Applications | Wide application in standard electrochemical cells, corrosion studies, and pH measurements. | Common in biomedical applications, particularly in physiological and biological research. Also, possesses a lower impedance, which makes it suitable for high-frequency applications. |