pH is a fundamental parameter that characterizes the acidity or alkalinity of a substance. For drinking water, pH plays a pivotal role in determining its quality and potential impact on human health. This article reviews the importance of pH in drinking water, the optimal pH range for drinking water, and its health effects.
Importance of pH in Drinking Water
The pH of drinking water is crucial for various reasons. It influences the taste, odor, and overall palatability of water. In addition, pH levels affect the solubility and bioavailability of minerals in water, which impacts its nutritional content. For example, at low pH levels (pH < 6.5), water is acidic, soft, and corrosive. This causes leaching of metal ions from the water pipe into the water supply, creating a more toxic composition that is harmful to drink. In addition, low pH creates aesthetic problems such as metallic or sour taste, staining of laundry, and staining on sinks and drains. Its corrosive nature also damages metal piping.
On the other hand, high levels of pH (pH > 8.5) also present some issues. The water may taste bitter or soapy. It can also result in scaling in pipes, reduction in water flow, and also increases the risk of corrosion. Extremely alkaline water can also interfere with normal functioning in the body and lead to health problems. In laundry applications, water at high pH makes detergents and soaps less effective, thus its name, hard water.
Understanding the pH of drinking water is also important for water treatment processes. Municipal water treatment facilities often adjust the pH of water to optimize the effectiveness of disinfection processes like chlorination. Proper pH control during water treatment helps prevent the formation of harmful disinfection byproducts and ensures the overall safety of the water supply.
Optimal pH Range for Drinking Water and pH Measurements
According to regulatory bodies such as the WHO and EPA, the optimal pH range for drinking water lies between 6.5 and 8.5. Within this range, water has a generally acceptable taste and odor, so it is considered safe for consumption. Deviations from this range may not only affect the palatability of water but also its potential health implications.
Typically, surface water has a pH value between 6.5 and 8.5, while groundwater tends to have a pH between 6.0 and 8.5. These are two major drinking water sources and largely fall within the recommended pH range. However, environmental factors and human activities can have a harmful effect on the pH value of these water sources. For example, the emission of sulfur dioxide and nitrogen oxides through industrial operations and vehicles can produce acid rain. This goes into drinking water sources and reduces the pH value. As a result, it is necessary to continuously monitor the pH and carry out some form of water treatment. Litmus paper, which changes color to indicate if a substance is acidic or alkaline, is a basic way of assessing pH. More accurate measurements involve the use of pH meters and pH electrodes to give the specific pH value.
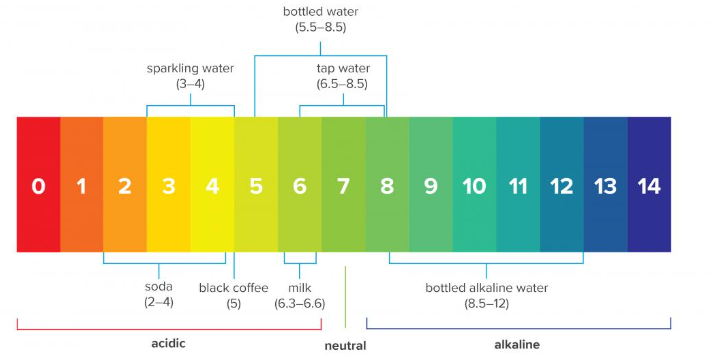
Consuming moderate amounts of slightly acidic liquids like sparkling water, soda, and black coffee do not result in harmful health effects.
Health Effects of pH in Drinking Water
The human body is constantly in the process of maintaining pH equilibrium, with the renal system and pulmonary system as the two main modulators. Thus, the consumption of water that is too acidic or too alkaline can have adverse effects on health. For example, acidic water can erode tooth enamel and lead to dental problems. Moreover, this type of water is associated with harmful concentrations of heavy metals such as copper and lead. Chronic exposure to elevated levels of these metals can contribute to neurological and developmental issues. Alternately, frequent consumption of excessively alkaline water can cause gastrointestinal issues like indigestion and constipation. However, research shows that there could be some benefits in consuming slightly alkaline water (pH between 8.5 and 10).
Alkaline Water
The consumption of alkaline water has become a trend over the past few years. There have been several claims of its benefits, such as slowing down aging, maintaining a healthy pH in the body, and blocking chronic diseases like cancer. It is also said to have antioxidant properties that reduce oxidative stress and protect cells from damage caused by harmful free radicals. Some other benefits of drinking alkaline water are as follows:
- Hydration and Detoxification: Water with a pH between 8.5 and 10 tends to have more minerals and electrolytes. Thus giving it a smaller cluster size. This plays a crucial role in improving cellular absorption and preventing dehydration in people who are sick or undergoing intense exercise. In addition, alkaline water is suggested to aid in detoxification and neutralizing of toxins from the body.
- Improving Digestive Health: The human stomach has a naturally low pH level of about 2, which is beneficial acidity that helps with food digestion. However, frequently consuming acidic food can lead to the build-up of this acid, making it rise in the esophagus. Thereby causing acid reflux or heartburn. Consuming alkaline water helps to neutralize this excess stomach acid and improve digestion. In addition, alkaline water is suggested as a good therapy for people with irritable bowel syndrome, especially those whose primary symptom is diarrhea.




