The pH level in food plays a crucial role in determining its taste, texture, safety, and shelf life. As a result, it is one of the foremost considerations in various aspects of food production, preservation, and quality control. This article delves into understanding the significance of pH in food, different methods for testing pH levels, a food chart, and applications in food processing and preservation.
The Significance of pH in Food
The pH level of a substance measures its acidity or alkalinity. In food, it serves as an indicator that governs key processing aspects, as the following sections highlight.
Microbial Growth and Food Safety
The pH level of food influences the growth of microorganisms, including pathogenic bacteria. Most bacteria thrive in neutral to slightly acidic environments ranging from pH 6 to 7. So, lowering the pH can inhibit the growth of pathogens like salmonella, listeria, and E. coli, which enhances food safety. Lowering the pH is key in several food preservation methods, such as pickling, fermentation, and canning.
Chemical Reactions and Food Quality
Another way pH impacts food is in its influence on enzymatic reactions. For example, enzymes involved in browning reactions in fruits and vegetables are active at specific pH ranges. So, controlling the pH can prevent undesirable browning and maintain the quality and appearance of food products. In addition, the Maillard reaction which occurs between amino acids and reducing sugars, which is responsible for browning and flavor development in baked goods and roasted meats, is pH-dependent. The optimal pH for this process is slightly alkaline, so adjusting the pH can enhance flavor and color development.
Flavor and Sensory Attributes
The pH level has a direct influence on the taste of food. Acidic foods tend to have a sour taste, while alkaline foods can taste bitter. So, in products such as sauces, dressings, and beverages, balancing the pH is essential in achieving a particular flavor profile. Also, pH impacts the texture of food products. A clear example is pH affecting protein coagulation and the final texture in dairy products like cheese and yogurt. Another is pH affecting the action of leavening agents in baked goods, which determines the crumb structure.
Nutritional Quality
Certain nutrients’ stability is pH-dependent. For instance, vitamin C is more stable in acidic conditions, while some B vitamins prefer neutral to slightly alkaline environments. This means adjusting pH can help preserve the nutritional quality of certain food products. Another area of impact is the bioavailability of nutrients. The pH of the digestive tract and the food matrix can influence the solubility and absorption of minerals like iron and calcium.
Shelf Life and Storage
Lowering the pH of food products prevents spoilage by inhibiting the growth of certain microorganisms and enzymatic activities that degrade food. This is important for perishable items like fruits, vegetables, and dairy products. Moreover, the pH of food products can determine packaging and storage requirements. For example, acidic foods need more robust packaging to prevent corrosion or chemical reactions that could compromise food safety.
Regulatory Compliance
Many food products are subject to regulatory standards that specify acceptable pH ranges to ensure safety and quality. For example, the FDA recommends a pH greater than 4.6 and water activity greater than 0.85 for low-acid canned foods. Acidified foods should have a pH of 4.6 or below, with water activity greater than 0.85. Also, accurate pH measurement and control are essential for compliance with labeling requirements, especially for low-acid and acidified foods.
Testing pH Levels of Food
Understanding pH’s impact in food shows the necessity of deploying various methods for testing pH levels. However, the food sample should be put in a state where it is possible to take accurate measurements.
Sample Preparation in Food pH Analysis
The food sample preparation techniques to improve pH measurement are as follows:
- Blending and Homogenization: Most food samples containing solids and semi-solids require blending to improve the accuracy of pH measurements. This blending should be such that the contents form a uniform paste. If the food consists of solids and liquids, then the sample’s solid-to-liquid ratio should represent the food. When blending, the sample cannot form a homogeneous paste: add 10 to 20 mL of distilled water per 100 grams of the sample and blend.
- Juicing: For fruits and vegetables, juicing the sample provides a liquid medium for accurate pH measurement.
- Dilution: Some concentrated or viscous samples may require dilution using a particular volume of distilled water. Generally, follow the same recommendation of adding distilled water as highlighted previously.
In addition, it is important to allow the sample to cool to about 25℃ before taking measurements. This is because at higher temperatures, ion dissociation increases, resulting in a drop in pH. So, taking pH measurement of hot food will give lower values than the actual equilibrium pH of the food.
pH Testing Methods
| Methods | Procedure | Advantages | Applications | Considerations |
| pH Pens | Insert probe into food sample to measure pH. | Provides good pH accuracy and is portable. | Ideal for liquids, semi-solids, and solids after sample preparation. | Requires regular calibration and maintenance. |
| pH Strips (Litmus Paper) | Paper changes color after insertion in food samples to indicate pH level. | Portable, inexpensive, and simple to use. | Serves for liquids and semi–solids. | Indicates if food is acidic or alkaline, but does not reveal exact pH value. |
| Colorimetric | Add a dye to the food sample, which results in a color change. Then, compare to a standard color chart to reveal the pH level. | Easy to use and cost- effective. | Liquids and semi-solids, especially for educational purposes, and preliminary testing. | Provides approximate values, so less accurate than pH electrodes, as well as pH pens. |
| pH Electrodes | Place electrodes in direct contact with food and read pH value from the meter. | Provides highest pH accuracy due to the various pH electrode designs available. | Suitable for direct placement in samples like meats, cheeses, and solid foods, whereas pH pens require sample preparation. | Best accuracy of all pH test methods with regular calibration and maintenance. |
pH in Food Chart
The ideal human blood pH is between 7.35 and 7.45, with studies showing optimal performance at 7.39. Although the food we eat is unlikely to change the body pH, there is a general consensus to maintain a balance in consumption. This is why pH food charts are quite popular for keeping track of common foods that we consume.
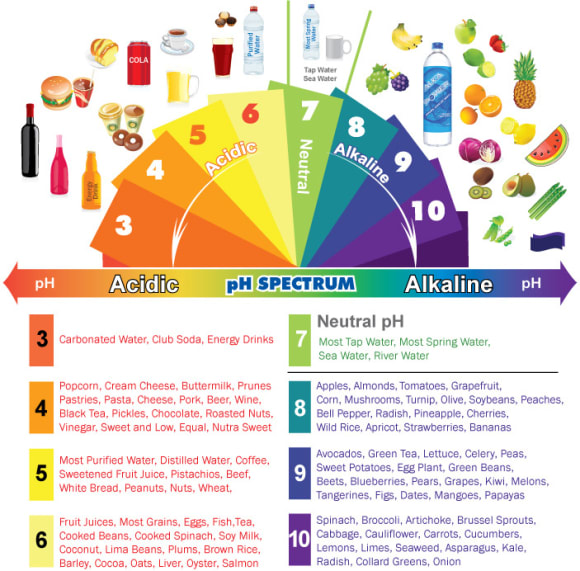
Generally, illnesses tend to accompany consuming high-acid foods (pH<4.6) excessively, more than alkaline foods. But this does not suggest that excessive consumption of high-alkaline foods is good. A balance is ideal to promote good health.
pH in Food Processing and Preservation
Understanding, testing, and controlling pH levels are crucial in various food processing and preservation methods as follows:
- Fermentation: In fermentation, beneficial bacteria convert sugars into acids, lowering the pH and preserving the food. Examples include yogurt, sauerkraut, and kimchi.
- Canning: The pH level determines the heat treatment requirements to ensure the destruction of pathogens. As a result, high-acid foods with pH below 4.6 require less heat treatment than low-acid foods.
- Dairy Production: Controlling pH is essential for protein coagulation and texture development. The pH level influences the final product’s firmness, flavor, and shelf life.
- Baking: Proper pH control ensures optimal rise and texture, because it affects the activity of leavening agents.
- Meat Processing: The pH level affects the water-holding capacity, tenderness, and shelf life of meat products
How to Selecting the Right pH Testing Method
At AlpHa, our portfolio of pH measurement devices including digital meters, electrochemical, and PTSA sensors. We are also available to discuss your specific measurement needs and design custom pH sensors for you. Contact us today to get started.




