An ion selective electrode is a unique sensor that measures the concentration of specific ions in a solution. It is also known as a specific ion electrode and membrane electrode. Because of its uniqueness, its application cuts across several fields, including environmental monitoring, medical diagnostics, and industrial processes. This article explores what an ion-selective electrode is, its types, and common examples like chloride, nitrate, and sodium.
What is an Ion Selective Electrode?
Ion Selective Electrodes (ISEs) enable the electroanalysis of a solution by measuring the activity of specific ions within the solution. They operate on the principle of selective ion exchange, thus allowing for determining ion concentrations with high specificity. Other advantages of this type of sensor include:
- Capability of detecting low concentrations.
- Also, rapid response to changes in ion concentrations.
- Applicability to a wide range of ions as well as sample types.
- Requires minimal sample preparation.
Before delving deeper into the measurement process, it is necessary to identify the key components of this sensor.
Components of an ISE
- Ion Selective Membrane: This is the core component that selectively interacts with the target ion. The type of membrane in any setup depends on the type of ion to be measured in the sample solution. For example, glass membranes are effective for H+ (pH electrodes), and crystalline membranes for F- (fluoride electrodes). In addition, solid-state membranes are ideal for Cl- (chloride electrodes) and polymeric membranes for NO3- (nitrate electrodes).
- Membrane Electrode: Typically, an Ag/AgCl (silver/silver chloride) electrode is behind the ion selective membrane and is in direct contact with a standard solution or membrane.
- Membrane Inner Fill Solution: This solution contains a particular concentration of the ion of interest, maintaining a stable internal environment.
- Reference Electrode: This is an important part of the setup as it completes the electrochemical cell. It is usually either a single-junction or double-junction refillable Ag/AgCl electrode type. Either way, it is always immersed in a reference electrolyte solution.
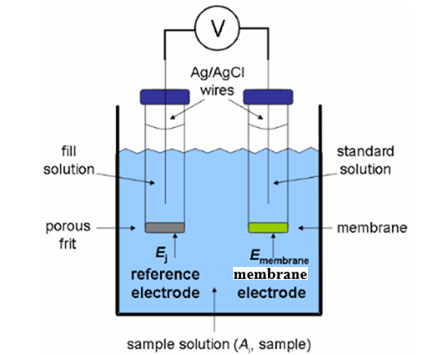
Ion Selective Electrode Working Principle
An ion selective electrode functions based on the Nernst equation. This equation describes the electrochemical potential due to the concentration of an ion on either side of a membrane. A breakdown of the measurement process is as follows:
- Selective Ion Exchange: After immersing the ISE in a solution containing the target ion, ions from the solution interact with the ion-selective membrane, thus changing the charge distribution across the membrane.
- Potential Development: The difference in ion concentration between the fill solution and the sample solution creates a voltage across the membrane. According to the Nernst equation, this potential is related to the ion activity in the solution.
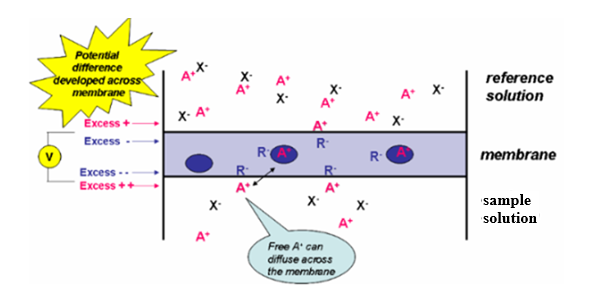
- Nernst Equation defines the voltage (E) across the membrane as a function of the standard electrode potential (E0). This voltage also depends on the universal gas constant (R), the temperature in Kelvin (T), and Faraday’s constant (F). Other influencing factors include the charge number of the ion (z) and the activity of the ion in the sample solution (asample), as well as fill solution (afill).
![]()
- Measurement: The potential difference is measured by the voltmeter or pH meter connected to the ISE and the reference electrode. The instrument then translates this potential into the ion concentration using the calibration curve from standard solutions of known concentrations.
Ion Selective Electrode Types
Ion Selective Electrodes (ISEs) come in various types, depending on the membrane type in use. Moreover, each membrane type suits the measurement of specific kinds of ions. The following sections highlight the most common types in the industry.
Glass Membrane Electrodes
This type of ISE utilizes special glass membranes formulated from materials such as silicate or chalcogenide. These glass membranes have excellent selectivity. Silicate glass compositions are specific for single-charged cations like H+, Na+, and Ag+. Chalcogenide glass has selectivity for double-charged metal ions such as Pb2+ and Cd2+. Glass membranes have excellent chemical durability so that they can work in very aggressive media. All these features make them an ideal choice for pH measurements, which is the most common use of glass membrane electrodes.
Solid State (Crystalline) Membrane Electrodes
Solid State membranes constitute a crystalline substance like LaF3 crystal or powdered compounds pressed into a solid pellet like AgI/Ag2S membrane. This membrane type has good selectivity because only ions that can permeate into the crystal or pellet structure can affect the electrode response. Examples include chloride, bromide, and iodide membrane electrodes, as well as copper, lead, and cadmium membrane electrodes.
Polymer Membrane Electrodes
This type of membrane is made of a polymer matrix containing a specific ionophore that selectively binds the target ion. Examples include valinomycin-based polymer for potassium and calcium ionophore-based polymer for calcium. Other commonly measured ions include magnesium, nitrate, and ammonium.
Gas-Sensing Electrodes
These are ideal for measuring gases that can dissolve in water and form ions such as ammonia, carbon dioxide, and nitrogen oxide. They consist of a gas-permeable membrane that allows gas to diffuse into the internal fill solution. Then, the internal solution and gas react to form hydrogen or hydroxide ions. Thus, a pH change occurs and is measured by an internal pH electrode positioned behind the membrane. As a result, this type of ion selective electrode does not require does not require an external reference electrode.
Ion Selective Electrode Examples
Ion Selective Electrodes (ISEs) for specific ions like chloride, nitrate, and sodium are essential tools in analytical chemistry. Moreover, these electrodes provide selective and accurate measurements of these ions in various solutions.
Chloride Ion Selective Electrode
The chloride ISE is usually of the solid-state membrane type with Ag2S/AgCl electrodes used to detect chloride ions in a solution. It could also be in the form of a polymer membrane containing an ionophore selective for chloride ions. Some common applications of chloride ISE are as follows:
- Clinical: Monitoring chloride levels in blood, urine, and other bodily fluids.
- Environmental: Measuring chloride in water bodies, wastewater, and soil.
- Industrial: Analyzing chloride in various industrial processes, including food and beverage production.
Nitrate Ion Selective Electrode
The nitrate ISE typically uses a polymer membrane containing an ionophore selective for nitrate ions. Upon immersion, the membrane interacts with nitrate ions, generating a voltage correlating with the ion concentration. They are common in the following applications:
- Agriculture: Monitoring nitrate levels in soil and fertilizer solutions to optimize plant growth.
- Environmental: Assessing nitrate pollution in water bodies and wastewater.
- Water Quality: Measuring nitrate levels in drinking water to ensure safety and compliance with regulations.
Sodium Ion Selective Electrode
The sodium ion selective electrode typically uses a glass membrane to measure the concentration of sodium ions in a solution. Common applications include:
- Medical: Monitoring sodium levels in blood, urine, and other bodily fluids for diagnosing and managing health conditions.
- Environmental: Measuring sodium in natural waters, wastewater, and soil.
- Industrial: Analyzing sodium content in various industrial processes, including food and beverage production and chemical manufacturing.
Choosing the Right Ion Selective Electrode
Ion selective electrodes are vital in analytical chemistry, providing precise and reliable measurements of specific ion concentrations in various environments. However, it is necessary to select the right type for your application to maximize the benefits of this sensor. At AlpHa, we offer a broad ISE portfolio from which you can choose either standard or customized sensors for your application. Contact us today, as our team can guide you in getting what is best for your project.



