Heavy metals in water pose significant risks to human health and the environment. Understanding their impact and how to handle them is crucial for ensuring safe and clean water supplies. This article explores the sources of heavy metals common in water, their effects, testing methods, and effective strategies for removal.
What are Heavy Metals
Heavy metals are a group of metals and metalloids with relatively high density, hence the name ‘heavy.’ Some of the most common metals are lead, mercury, tin, and silver, which serve in several applications. Because of their predominance, it is common to find heavy metals in various aquatic environments and eventually in living organisms that depend on these water resources. Many of these metals are essential to human and plant functions, but they can be toxic in large doses.
Sources of Heavy Metals in Water
Heavy metal pollution in water resources can arise from both natural and anthropogenic (human-induced) sources. The following sections highlight some of the common sources of pollution.
Industrial Discharges
- Mining: Mining activities could release heavy metals such as mercury, lead, and arsenic into nearby water bodies.
- Metal Processing and Manufacturing: Metal refining, electroplating, and battery manufacturing generally discharge metals like cadmium, chromium, and nickel into water sources.
- Textile and Tanning Industries: These industries use heavy metals like chromium and lead in dyes and chemicals, thus leading to pollution.
- E-waste Recycling: Improper electronic waste recycling can lead to heavy metals like lead and mercury entering water sources.
Agricultural Runoff
- Fertilizers and Pesticides: Many agricultural chemicals contain heavy metals like arsenic and cadmium. During rainfall or irrigation, these metals seep into the soil, build up in volume, and eventually leach into water bodies.
- Animal Manure: Livestock feed often contains heavy metals like zinc and lead, which can enter water sources through runoff from fields treated with manure.
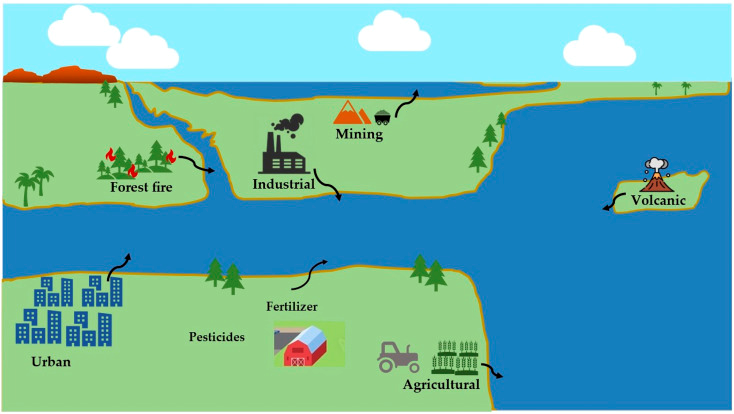
Urban Runoff
- Road Runoff: Vehicles contribute to heavy metal pollution through tire wear (zinc and cadmium) and brake lining wear (copper and antimony).
- Stormwater: Urban areas can contribute to heavy metal pollution through stormwater carrying metals from buildings, streets, and construction sites.
Waste Disposal
- Landfills: Leachate from improperly managed landfills can carry heavy metals into groundwater and surface water.
- Incineration: Emissions from waste incineration can deposit heavy metals into water bodies through atmospheric deposition.
- Household Products: Some household products, like paints, batteries, and cosmetics, contain heavy metals that can enter water sources through wastewater systems.
Natural Sources
- Weathering of Rocks: Natural weathering of metal-bearing rocks releases heavy metals like arsenic and chromium into water bodies.
- Volcanic Activity: Volcanoes can release heavy metals into the environment, entering water systems.
Effects of Heavy Metals in Water
Several heavy metals are on the World Health Organization’s list of Chemicals of Major Public Health concerns. Some of them include lead, cadmium, mercury, and arsenic, all common in tap water.
Lead
According to the WHO, the permissible limit for lead is 0.01 mg/L (ppm). Exposure to lead above this limit could result in cognitive deficits, learning disabilities, and behavioral problems, especially in children. Other consequences on human health include hypertension and heart disease, while chronic exposure can cause kidney dysfunction. Lead can also accumulate in soil and water bodies, adversely affecting plant and fish growth.

Mercury
Mercury beyond the permissible limit of 0.001 mg/L (ppm) can cause severe neurological damage and developmental delays in fetuses and young children. In addition, mercury exposure can weaken the immune system and lead to renal impairment or eventual failure. In wildlife, high mercury levels can disrupt reproductive and behavioral patterns.
Cadmium
Long-term exposure to cadmium above 0.05 mg/L (ppm) can damage kidneys and cause bone demineralization, thus increasing the risk of fractures. Cadmium is also a carcinogen linked to lung and prostate cancer. Like other heavy metals, cadmium can contaminate soil and water, adversely affecting plant and aquatic life.
Arsenic
Arsenic in amounts higher than 0.01 mg/L (ppm) can cause skin lesions and hyperpigmentation. It can also increase the risk of heart disease and is a known carcinogen with links to skin, lung, bladder, and liver cancers. High arsenic levels in soil and groundwater inhibit plant growth and agricultural productivity.
Chromium
Chromium is naturally present in many foods and also plays a crucial role in sugar and fat metabolism. Its deficiency can cause insulin resistance, which leads to diabetes and heart disease. However, inhalation in excess amounts above 0.003 mg/L (ppm) can cause lung inflammation, asthma, and even lung cancer. Also, direct contact can cause skin ulcers and dermatitis.
Methods of Removing Heavy Metals from Water
| Method | Process | Advantages | Disadvantages |
| Chemical Precipitation | Adding chemicals to form insoluble compounds. | Targets heavy metals which are difficult to remove and is a very selective removal | Sludge generation, as well as disposal issues. Also high cost and energy intensive. |
| Ion Exchange | Using resins to exchange heavy metal ions. | Highly efficient, selective removal. | Requires regular regeneration and expensive resins. |
| Adsorption | Adsorbing metals onto materials like activated carbon. | Effective for low concentrations, low-cost. | Slow process, costly replacement/regeneration of adsorbents. |
| Membrane Filtration | Using semi-permeable membranes, for example, reverse osmosis. | High removal efficiency, produces high-quality water with complete demineralization. | Frequent membrane replacement with high costs. |
| Electrochemical Treatment | Using electrical current for coagulation or plating. | Effective on a wide range of contaminants. | High energy consumption and complex operation. |
| Bioremediation | Using plants or microbes to absorb, as well as detoxify metals. | Environmentally friendly. | Slow process with variable effectiveness. |
| Coagulation and Flocculation | Adding coagulants to aggregate particles. | Cost-effective, widely used. | Chemical sludge production and disposal issues |
| Phytoremediation | Using hyperaccumulator plants. | Eco-friendly, low-cost. | Slow process, limited to suitable plant growth areas. |
| Advanced Oxidation Processes | Generating reactive species to oxidize metals. | Effective for wide range of contaminants, complete mineralization. | High cost, handling of oxidizing chemicals. |
How to Test for Heavy Metals in Water
| Method | Principle | Advantages | Disadvantages |
| Atomic Absorption Spectroscopy (AAS) | Measures light absorption by metal atoms. | Highly sensitive as well as accurate. | Very expensive equipment and also requires skilled operators. |
| Inductively Coupled Plasma Mass Spectrometry (ICP-MS) | Ionizes sample as well as detects ions based on mass-to-charge ratio. | Extremely sensitive, multi-metal detection. | Costly equipment and also requires skilled operators. |
| Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) | Measures light emission from excited atoms. | High-sensitivity, relatively low-cost equipment. | Expensive equipment and skilled operators needed. |
| X-Ray Fluorescence (XRF) | Measures secondary X-rays emitted by excited atoms. | Non-destructive, rapid, field use possible. | Limited sensitivity, interference from other elements. |
| Electrochemical Methods | Measures current response to applied potential. | High sensitivity, relatively low-cost equipment. | Requires calibration, potential interference. |
| Colorimetric Methods | Measures color change from reagent-metal reaction. | Simple, low-cost, field use possible. | Limited sensitivity and specificity, interference possible. |
Testing for Heavy Metals with ANDalyze
AlpHa Measurement Solutions offers the ANDalyze handheld fluorometer, a proprietary catalytic DNA sensor capable of real-time analysis of heavy metals. Moreover, ANDalyze leverages cutting-edge DNAzyme technology to deliver quick and reliable results without requiring extensive chemical knowledge or handling hazardous materials. Key features of this product include:
- Rapid Detection: The ANDalyze fluorometer provides quantitative results in less than one minute, ensuring timely and efficient water analysis.
- Versatility: It measures multiple heavy metals, including lead, copper, uranium, mercury, cadmium, as well as zinc. Hence, making it suitable for drinking water, wastewater, surface water, food safety, and mining applications. It also covers a wide variety of parameters as the following table highlights.
| Sensor | Detectable Range | Accuracy | Color |
| Lead | 2 – 100 ppb | Greater of 15% or 2 ppb | Green |
| Uranium | 2 – 60 ppb | Greater of 15% or 2 ppb | Orange |
| Copper | 0.1 – 2 ppm | Greater of 15% or 0.05 ppm | Blue |
| Mercury | Pass/Fail at 2 ppb (up to 50 ppb) | N/A | Silver |
| Zinc | 1 – 15 ppm | Greater of 20% or 1 ppm | White |
| Cadmium | 0.1 – 1 ppm | Greater of 15% or 0.1 ppm | Yellow |
- Ease of Use: The device is designed for simplicity, requiring no special skills or chemistry knowledge, thus minimizing training and operational costs.
- Portability: Its handheld design allows for on-site testing, providing flexibility as well as convenience in various field conditions.
- EPA Validated: The technology is validated by the Environmental Protection Agency (EPA), hence ,ensuring reliability and regulatory compliance.